The treatment of atopic dermatitis in canine patients has been completely transformed by the introduction of oclacitinib, which was approved in 2014. Oclacitinib, which is a powerful Janus kinase inhibitor, is a novel targeted approach that suppresses the itching-inducing pathways that are the root cause of inflammatory skin conditions in dogs. Additionally, the development and production of its active pharmaceutical ingredient, oclacitinib maleate, are essential to the clinical success of this product.
The properties of oclacitinib maleate active pharmaceutical ingredients (APIs), as well as their formulations and production at commercial scale, are investigated in this article.
This is about the oclacitinib API. The synthetic compound Cyclohexanemethanesulfonamide, N-methyl-4-(methyl-3H-pyrrolo2,3-dpyrimidin-4- ylamino)-, trans-, (2Z)-2-butenedioate is the source of oclacitinib Maleate, which is a salt form of the compound. A methylated pyrrolopyrimidine core is connected to a methanesulfonanilide moiety through the use of an amino substituent. This is the structure that it takes on at the molecular level. This substance has a molar mass of 453.513 grams per mole and a chemical formula of C19H27N5O6S. Important characteristics of oclacitinib maleate active pharmaceutical ingredient include a low solubility in water but a high solubility in polar organic solvents. It has a powdered appearance that is characteristic of crystalline material that ranges from pure white to off-white in color, and it demonstrates stability for more than two years when it is packaged appropriately and exposed to ambient conditions.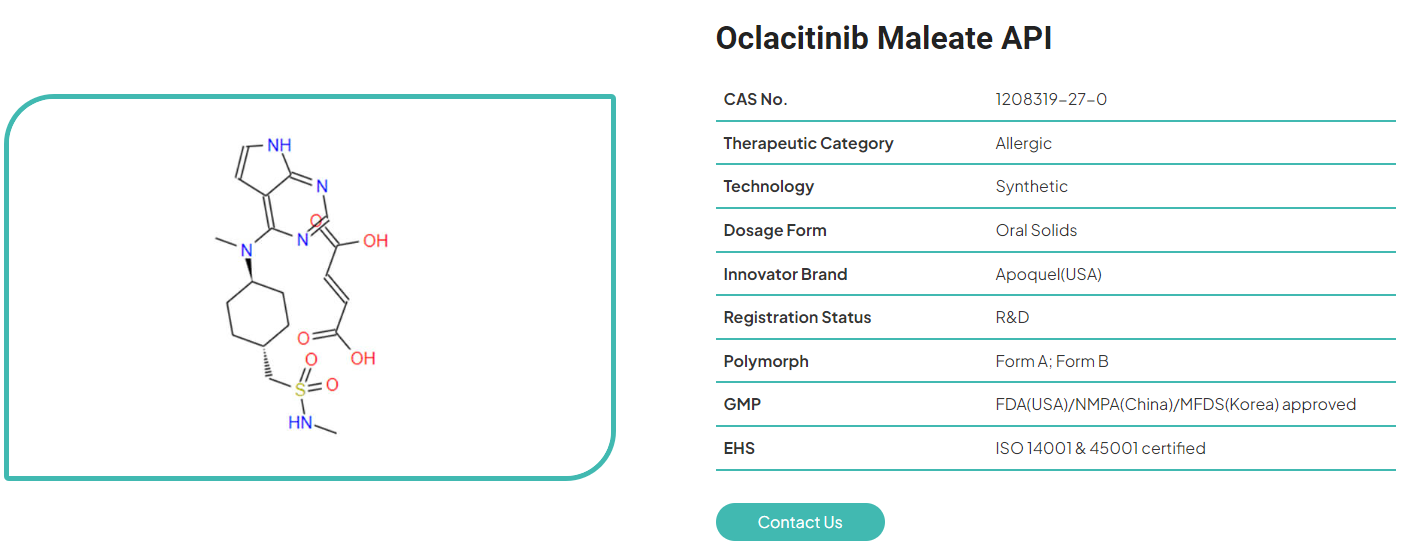 With these characteristics, formulated drug products are able to achieve a pharmacological effect that is reproducible over the course of their shelf life.
With these characteristics, formulated drug products are able to achieve a pharmacological effect that is reproducible over the course of their shelf life.
Veterinary Ingredients That Have Been ApprovedIn the only dermatological medication that has been approved for use on small animals, Apoquel® tablets contain oclacitinib API active pharmaceutical ingredient (API). Oral administration of Apoquel is recommended for canine patients, and the medication is available in film-coated tablet strengths of 5 mg and 20 mg. It is recommended that 0.4-0.6 mg/kg be administered twice daily, with the dosage being determined by the weight of the pet, which is measured in pounds, in order to prevent overdosing. When administered in accordance with the manufacturer's instructions, Apoquel has been shown to be both safe and effective in controlling pruritus and dermatitis in dogs that have atopic dermatitis.
Manufacturing on a Large Scale of Oclacitinib Maleate Active Pharmaceutical Ingredient
Qingmupharm. com, a market leader in the production of veterinary active pharmaceutical ingredients (API), has developed a proprietary technology that has been optimally optimized for the Oclacitinib manufacturer on a commercial scale. Multiple tons of active pharmaceutical ingredients (API) are produced annually in batches of high purity that consistently meet global compendial standards. Their production is compliant with cGMP and adheres to ICH, OECD, and local regulations. Qingmupharms' regulated production flow for oclacitinib maleate API includes a number of important aspects, including the following:Chemical synthesis of the active methylpyrrolopyrimidine subunit using an asymmetric approachComposition of the sulfonamide moiety that is attached through a multi-step coupling and purification sequenceIn order to isolate API, crystallization under vacuum or fluidized bed drying is performed. Tests for quality control that are above and beyond the acceptance criteriaFilling machines that are located within separate cleanrooms
The ability to trace items and comply with transportation regulations via packaging
Through the implementation of manufacturing excellence across integrated facilities, Qingmupharm guarantees the availability of Oclacitinib Maleate API on a global scale, thereby facilitating commercialization and clinical evaluation programs throughout the world. As a one-stop shop, they make it possible to expedite the development of compounds, from the feasibility study to the filing of regulatory files.
Factors to Consider When Manufacturing an Emerging Active Ingredient
A consistent and high-quality oclacitinib maleate active pharmaceutical ingredient was the foundation for the global commercialization of Apoquel after it was approved. Apoquel was the first inhibitor of its class to be approved. A manufacturing process that was optimized, GMP-compliant, and delivered at commercial scale in a cost-effective manner was required in order to meet the growing clinical demand. These kinds of production challenges are examples of the ones that innovator API manufacturers face on a regular basis in order to facilitate the development of new therapeutics.
The reason that API manufacturers play an unsung role in enabling medical breakthroughs through scalability and regulatory compliance is demonstrated by the fact that they are shown here. Patients all over the world are given the ability to benefit from their solutions, which help translate scientific discoveries into accessible cures. From the very beginning, developing robust manufacturing and quality control in accordance with the expectations of global health authorities presents a unique set of demands for emerging active pharmaceutical ingredients (APIs). In order to facilitate approval pathways in an efficient manner, suppliers are required to incorporate regulatory guidance in a proactive manner. Since 2012, Qingmupharm has been addressing these realities for the production of oclacitinib maleate.
This was accomplished by expanding their veterinary infrastructure and obtaining certification from multinational organizations. As a result of their continued success, research into new applications of JAK inhibition for animal and possibly human health applications is being made possible.
Veterinary API Supply Partner Role in the Workplace
With the introduction of Oclacitinib Maleate, Qingmupharm further solidifies its position as a leading veterinary Oclacitinib manufacturer. For more than a decade, Qingmupharm has been a reliable partner in the process of commercialization and development. Their products and services provide solutions to sourcing problems throughout the value chain:A consistent supply to global markets is made possible by mass production that can be scaled up to kilograms. API of the highest purity, manufactured in accordance with ISO 14001 certifications for the betterment of the environmentRegulatory experience helps to streamline the preparation of dossiers and provides support for pre-approval. Pricing that is 15-30% lower than that of competitors through the use of a direct bulk sales modelWe are able to facilitate dependable international deliveries within five business days thanks to our warehouse network. Expertise in formulation through research and development programs and procedures for process improvementIt is through the combination of large-scale capabilities and personalized solutions that Qingmupharm has established itself as the fully-integrated supplier partner of choice. Their dedication drives forward the ongoing progress that is being made to improve therapeutics for companion animals.

