Section 321 allows shipments valued at $800 or less to enter the U.S. duty-free, simplifying customs procedures for low-value imports. However, when it comes to FDA-regulated goods, an FDA Prior Notice must be submitted to ensure the shipment meets safety standards before arrival.The combination of Section 321 benefits with the FDA Prior Notice web entry process helps importers avoid delays and maintain compliance with both customs and FDA requirements. Understanding how these two systems interact is essential for smooth import operations.Many importers struggle with the complexities of the Prior Notice submission through the FDA’s web portal, but mastering this process can significantly reduce clearance times. This article breaks down what Section 321 entails and how to navigate FDA Prior Notice effectively.
Understanding Section 321 Customs and FDA Prior Notice Web Entry
Section 321 permits certain imported goods to enter the U.S. without formal customs entry, reducing paperwork and fees. FDA prior notice requires that the agency be informed of shipments containing food, ensuring safety compliance before arrival. These processes work together to facilitate faster clearance of qualifying imports.
Overview of Section 321 Customs Regulations
Section 321 of the Tariff Act of 1930 allows importers to bring goods valued at $800 or less into the U.S. duty-free and without formal customs entry. This provision applies only to goods intended for personal use or commercial purposes, but must not be part of a continuous shipment exceeding the value limit.Importers must file an electronic declaration with U.S. Customs and Border Protection (CBP). This declaration includes shipment details such as value, description, and origin. Section 321 reduces costs and time for low-value imports but has strict eligibility rules to prevent misuse.
FDA Prior Notice Requirements for Imports
The FDA mandates prior notice for all food shipments entering the U.S. This notification must be submitted electronically before the shipment arrives, allowing the FDA to assess risks and conduct necessary inspections.Prior notice includes product details, shipment dates, consignee information, and importer identity. Failure to provide accurate prior notice can result in shipment delays, refusals, or penalties. It applies to all food products, whether commercial or personal imports.
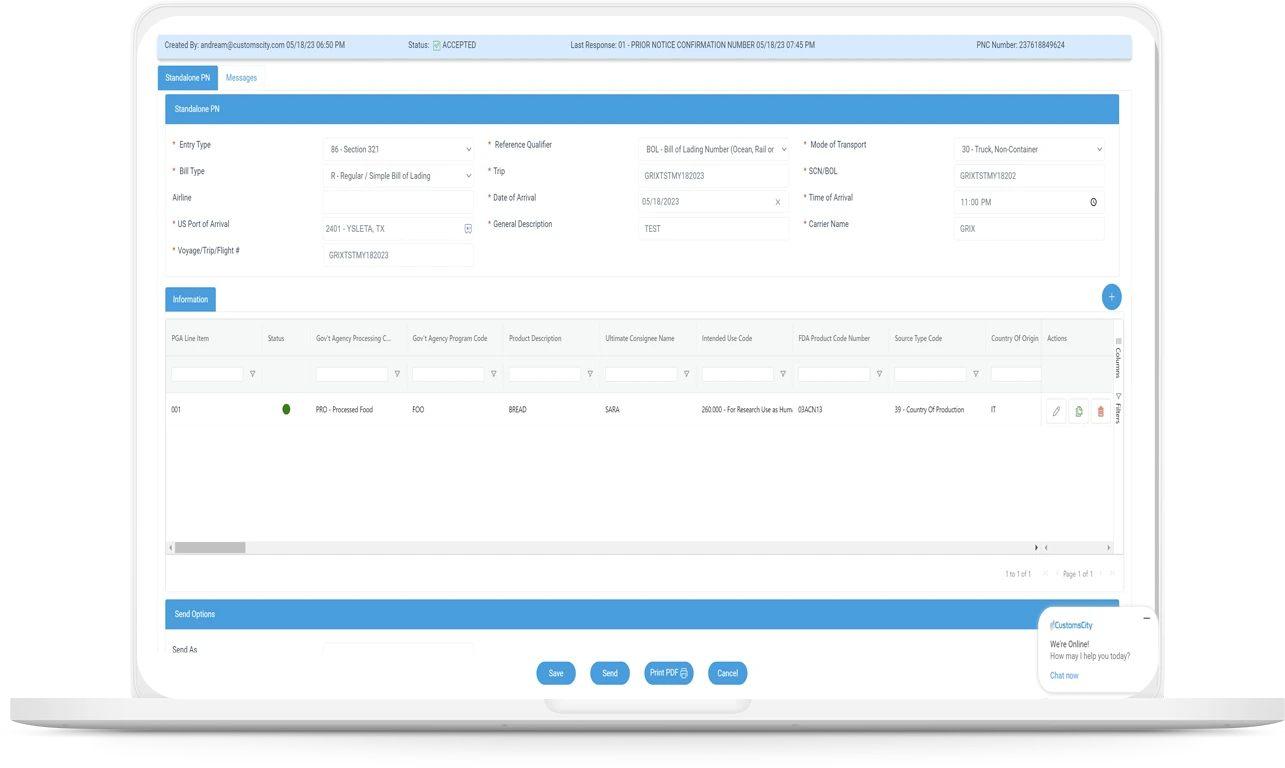
How Prior Notice Web Entry Streamlines Compliance
Web entry systems enable importers to submit FDA prior notice and CBP Section 321 declarations electronically in one unified platform. This reduces duplication, errors, and administrative delays during import processing.The system provides a digital record of submissions with confirmation receipts, improving traceability. Importers benefit from faster clearance, simplified compliance, and easier management of multiple shipments under Section 321 rules.
Key Differences Between Section 321 and Other Entry Types
Section 321 is distinct because it applies only to low-value shipments ($800 or less) that do not require formal entry paperwork or duties. Other entry types, like formal or informal entries, apply to higher-value or restricted goods and involve more complex customs processes.Unlike formal entry, Section 321 does not require customs bonds or entry summaries. However, both formal and informal entries may still require prior notice if they involve food products. Section 321 focuses on reducing regulatory burdens on small shipments while maintaining compliance.
Filing Procedures and Best Practices for Section 321 and FDA Prior Notice
Accurate submission of Section 321 declarations and FDA Prior Notice entries is essential for smooth customs clearance. Timely filing, proper documentation, and awareness of common mistakes reduce delays and compliance risks.
Step-by-Step Guide to Submitting Prior Notice Web Entry
The FDA Prior Notice must be submitted electronically before the arrival of imported food products. The filer logs into the Prior Notice System Interface (PNSI) or uses an Integrated Customs Broker.
Key data fields include:
- Importer and facility information
- Shipment details (arrival date, port of entry)
- Product description and quantity
- Manufacturer, grower, or shipper details
Submission must occur at least 2 hours before arrival by land or 4 hours before by sea/air. After submission, the system generates a Prior Notice Confirmation Number, which must accompany the shipment.Timely entry and accuracy ensure compliance and reduce the risk of shipment holds or refusals.
Common Errors and How to Avoid Them
Common errors include incorrect product descriptions, inaccurate arrival dates, and missing importer identification.Misreporting the shipment date can cause rejection or delays. Always confirm the port of entry and mode of transportation aligns with shipping documents.Duplicating submissions for the same shipment wastes resources and may trigger audits. Use the system’s search feature to verify prior filings.Errors in manufacturer or grower information lead to compliance issues. Verify all party names and addresses against reliable sources before submitting.Using a checklist for all required fields helps reduce omissions and inconsistencies.
Recordkeeping and Documentation Essentials
Maintain digital and physical copies of all Prior Notice confirmation numbers, Section 321 declarations, and supporting shipment documents.Records must be retained for at least two years from the date of entry. These include invoices, bills of lading, and correspondence with CBP and FDA.Proper organization speeds up responses to FDA or CBP inquiries and supports audits or reconciliation.Use secure systems with backups to avoid data loss. Consistent recordkeeping practices minimize compliance risks and demonstrate due diligence.